a)Ethyl acetoacetate(CH3COCH2COOCH2CH3) reactions with 1)NaoEt 2)CH3CH2Br 3)NaOH/H2o 4)H3o+ b)2Ethyl acetate (2CH3COOEt) reaction with NaOEt/H3o+ c)Diethyl malonate (CH2 (COOEt)2 )reaction with 1)NaoEt 2) CH3CH2Br 3)H3O+
Reaction 1:Ethyl acetoacetate reactions with 1)NaOEt 2)CH3CH2Br 3)NaOH/H2O 4)H3O+
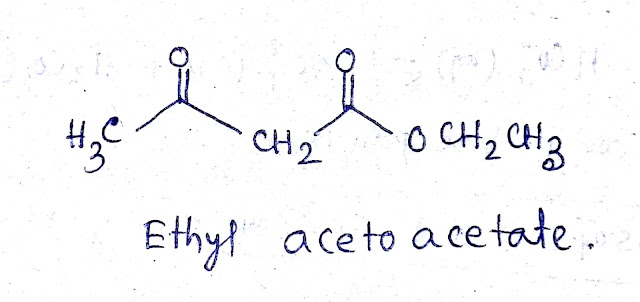
The structure of ethyl acetoacetate is-
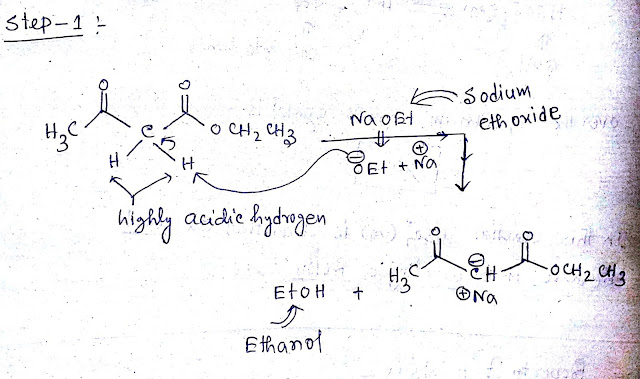
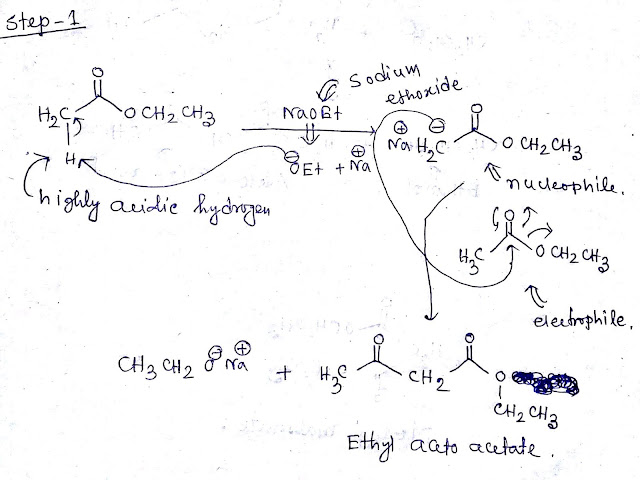
Step1:-Ethyl acetoacetate have highly acidic hydrogen due to presence of carbonyl group and ester group.When ethyl acetoacetate treated with strong base NaOEt this acidic proton will be captured by NaOEt as a result sodium salt of ethyl acetoacetate and ethanol be formed.The mechanism is shown below-
Step 2:-Sodium salt of Ethyl acetoacetate acts as a nucelophile and when ehtyl bromide (CH3CH2Br)added it take part in substitution reaction as a result CH3COCH (CH2CH3)COOEt will be formed.the mechanism is shown below -
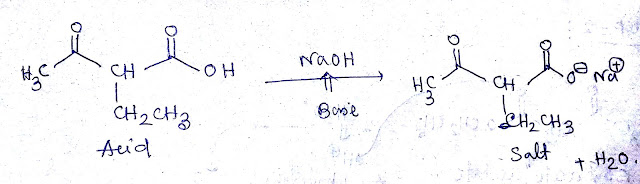
Step 3&4:-When CH3COCH (CH2CH3)COOEt treated with NaOH/H2O then hydrolysis of ester by base occur as a CH3COCH (CH2CH3)COONa will be formed.Then addition of H3O+ the product is CH3COCH (CH2CH3)COOH.The mechanism is shown below -
Reaction2:Two molecules of ethyl acetate (CH3COOCH2CH3) reacted with 1)NaOEt 2)H3O+
The structure of ethyl acetate is-
Step1:-Due to presence of ester group ethyl acetate(CH3COOCH2CH3) has highly acidic hydrogen and upon treated with strong base NaOEt this acidic proton will be captured and as a result a nucelophile(NaCH2COOCH2CH3) will produced.So one ethyl acetate molecule act as a nucelophile which attack the carbonyl carbon of ester group of other molecule as a result CH3COCH2COOCH2CH3 Produced.
Step 2:When NaCOOCH2COOCH2CH3 treated with H3O+ acid hydrolysis occur and HCOOCH2COOCH2CH3 Will be formed.The mechanism is shown below -
Reaction 3:Diethyl malonate (CH2 (COOEt)2) reacted with 1)NaOEt 2)CH4CH2Br 3)H3O+
The structure of diethyl malonate is-
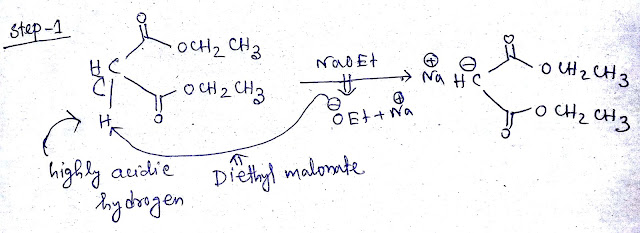
Step1:-Diethyl malonate (CH2 (COOEt)2) have highly acidic hydrogen due to presence of two ester group.When diethyl malonate treated with strong base NaOEt this acidic proton will be captured by NaOEt as a result sodium salt of diethyl malonate (NaCH (COOEt)2)and ethanol be formed.The mechanism is shown below -
Step 2:-NaCH (COOEt)2 acts as a nucelophile and treated with CH3CH2Br and substitution occur as a result CH3CH2CH (COOEt)2 will be formed.The mechanism is shown below -
Step 3:-Upon addition of H3O+ with CH3CH2CH (COOEt)2 hydrolysis of ester occur as a result CH3CH2CH2COOH will be formed.The mechanism is shown below -
Related questions


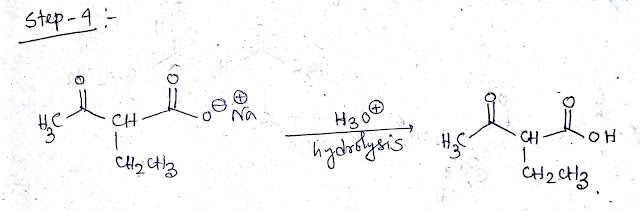


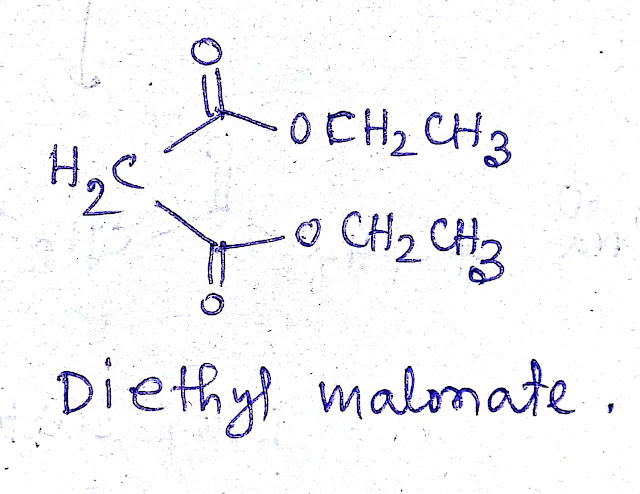





Comments
Post a Comment
If you have any quary regarding this blog please comment here.