Calculate the pH of a solution prepared by dissolving 1.30 g of sodium acetate, CH3 COONa, in 50.0 mL of 0.10 Macetic acid, CH, COOH(aq). Assume the volume change upon dissolving the sodium acetate is negligible. Ka of CH3COOH is 1.75 x 10 -5.
A mixture of weak acid here acetic acid and its salt acts as a buffer solution.
Buffer solution is a solution where addition of small amount of acid or base not change the pH.
The formula of ph for such solution is given by HENDERSON.

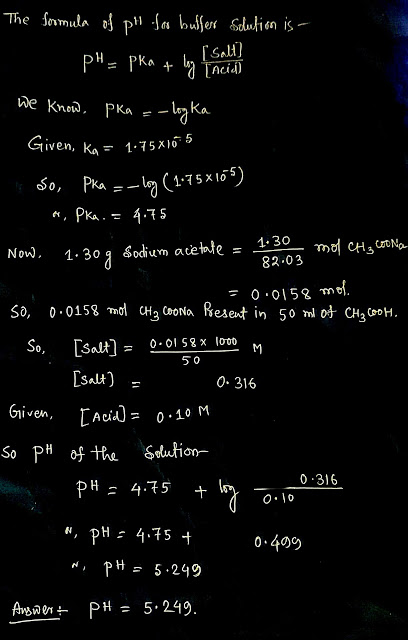



Comments
Post a Comment
If you have any quary regarding this blog please comment here.