Toluene(Ph CH3) reaxts with1) NBS/hv,2)Pph3,Buli and 3)Acetone
This is organic reaction with different reagent in sequence.
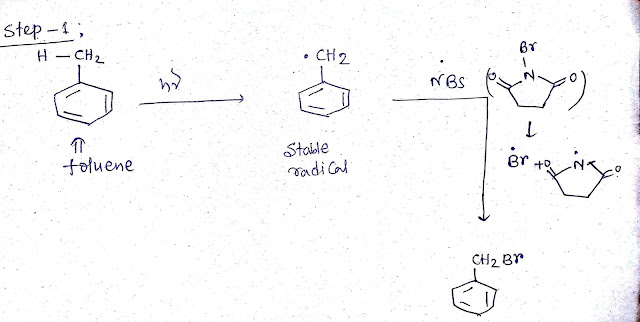
Step 1: Toluene (C6H6 CH3/PhCH3)reacts with NBS in presence of light (hv)
This reaction is known as allylic bromination.It is a free radical reaction where free radical is formed in presence of light.This allylic free radical is highly stable because it take part in resonance with benzene ring.Then brominating agent NBS provide bromine to this radical and thus bromination occur.
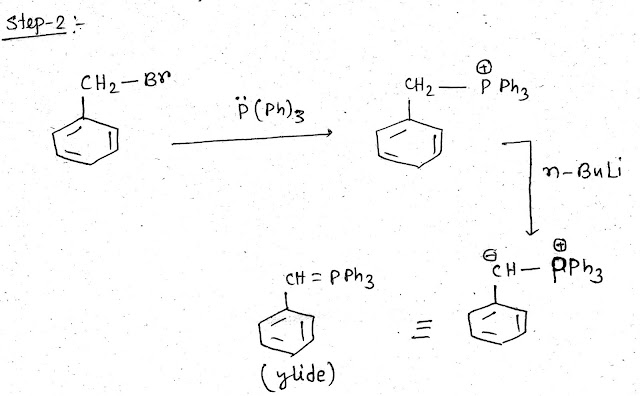
Step 2: Ylide (wittig reagent ) preparation
To prepare Ylide phenyl methyl bromide (PhCH2Br) with triphenyl phosphine P(Ph)3 in presence of strong base such as n butyl lithium (n BuLi)-
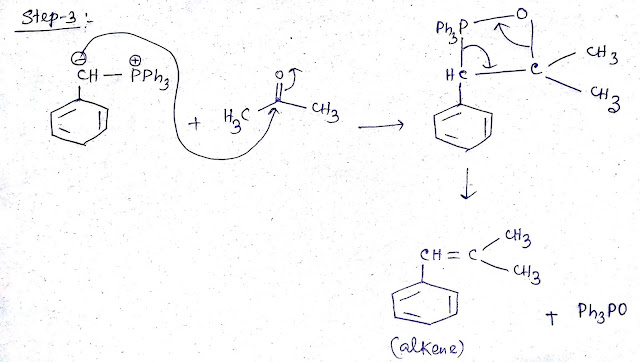
Step 3: Wittig reaction with prepared Ylide and acotone
Ylide carboanion acts as a nucelophile and attack electrophilic carbonyl carbon and a four memberd ring formed and a alkene formed as a product.



Comments
Post a Comment
If you have any quary regarding this blog please comment here.