Calculate the pH for the cell at 25°C-pt (s)|H2 (g,1bar)|H+(pH=?)||AgCl (s),Ag (s)|Cl-(aq,1M).Given Ecell=0.245v
Q.Calculate the pH for the cell at 25°C-
pt (s)|H2 (g,1bar)|H+(pH=?)||AgCl (s),Ag (s)|Cl-(aq,1M).Given Ecell=0.245v
Answer
Step 1:E°cell CalCulation
For this cell the cell reaetion is
H2+2AgCl=2H+ +Ag +Cl-
Thus E°cell=E°Agcl,Ag|cl- -E°H+/H2
=0.22 -0.00
=0.22v
Step 2:E cell CalCulation
To calculate Ecell we use nernst equation
Ecell=E°cell-(0.059÷n)log[H+]^2 [Cl-]
=0.22+0.059 pH
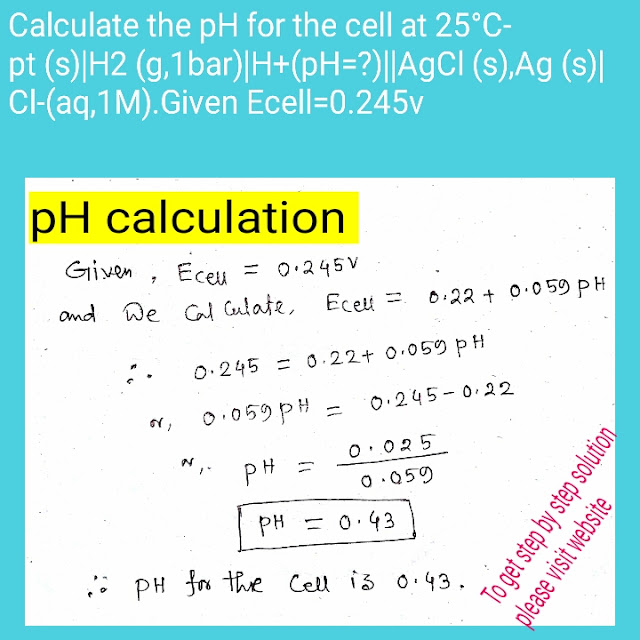
Step 3:pH calculation
Given Ecell=0.245v and we calculate
E cell=0.22+0.059pH
So,0.22+0.059pH=0.245
PH=0.43
Step 1:
Step 2
Step 3





Comments
Post a Comment
If you have any quary regarding this blog please comment here.